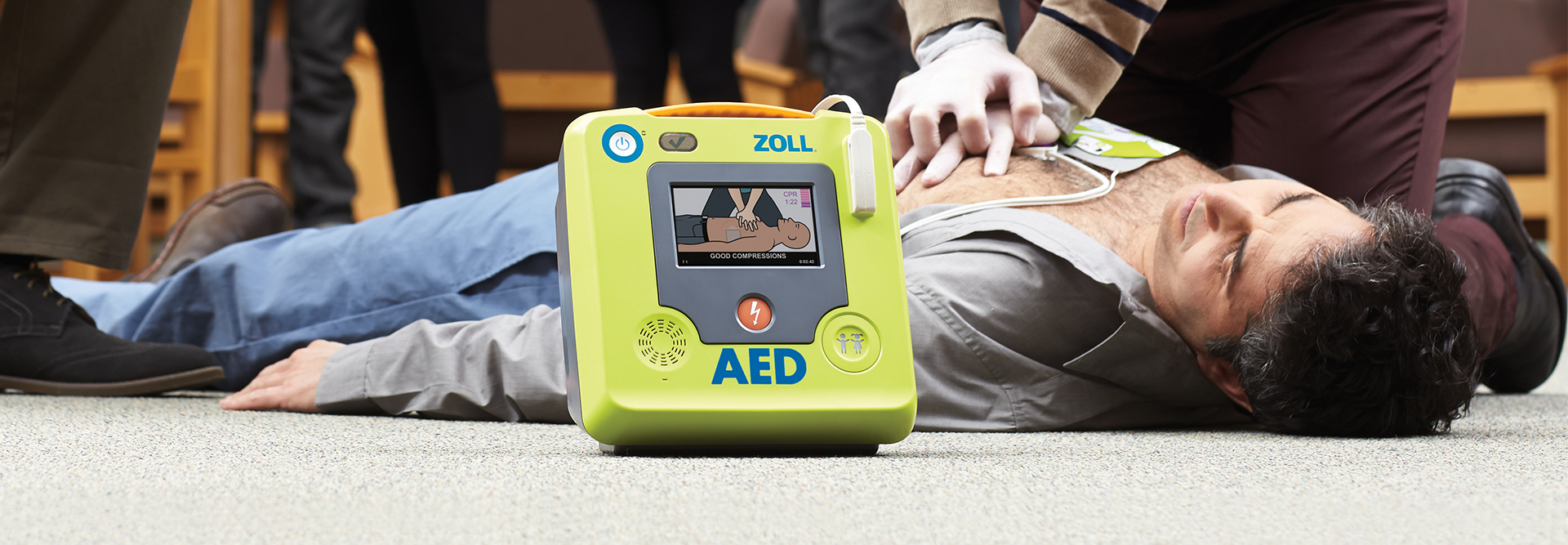
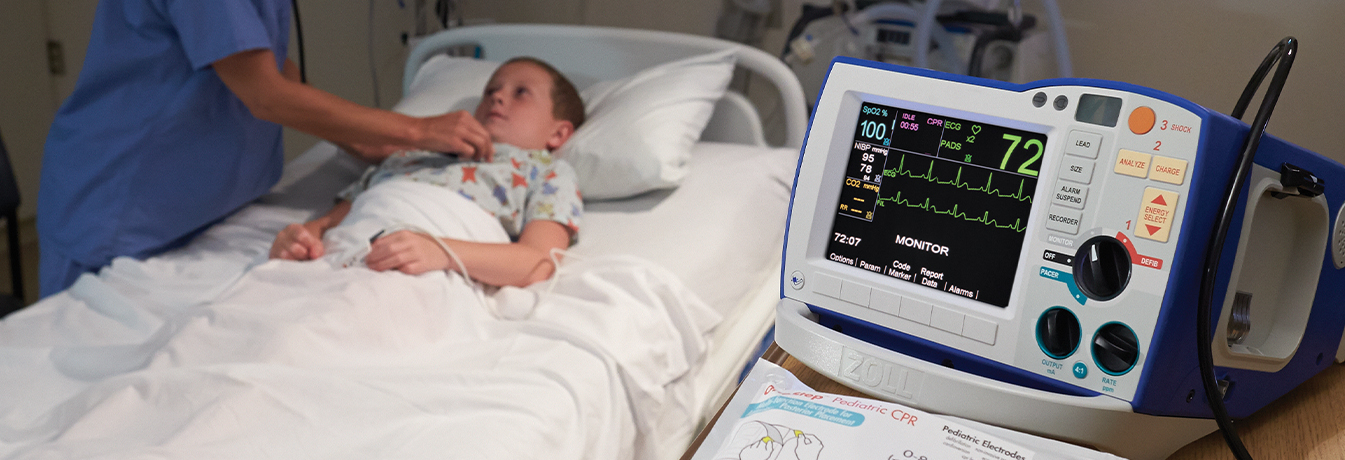
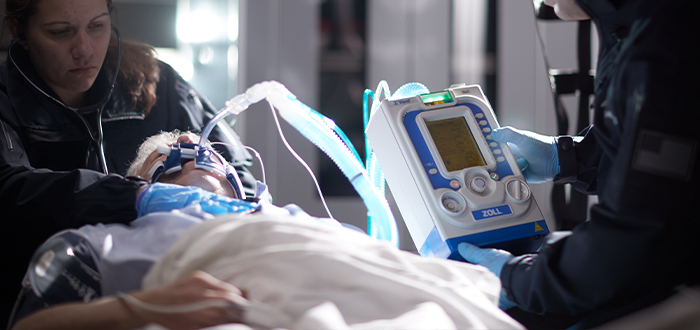
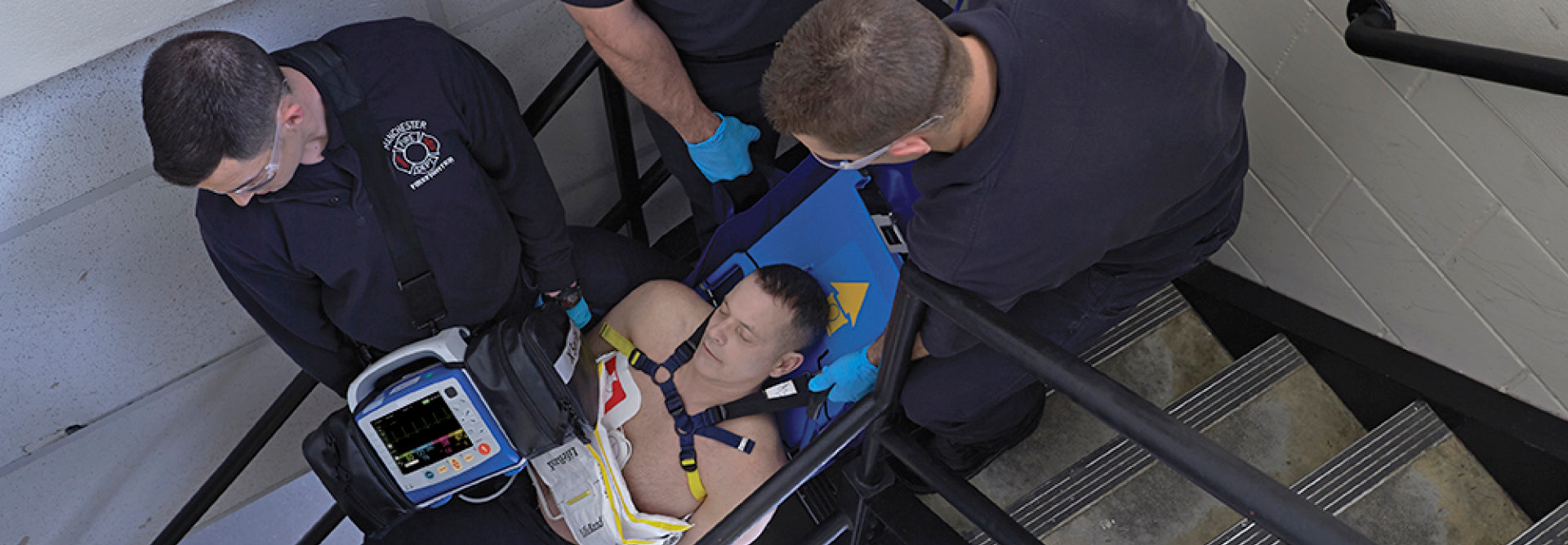
Resuscitation and Acute Critical Care Solutions
ZOLL® is focused on improving outcomes with novel resuscitation and acute critical care technology. Our medical products and software solutions help clinicians, EMS and fire professionals, lay rescuers, and the military provide life-saving care every day. ZOLL delivers technology that advances emergency care and benefits patients.